The U.S. Supreme Court unanimously rejected on Thursday a bid by a group of antiabortion doctors that would have potentially blocked widespread access to a common abortion pill.
In the opinion, written by Justice Brett Kavanaugh, the court determined that the group had no legal standing to challenge the U.S. Food and Drug Administration’s (FDA) regulation of mifepristone.
Here’s what to know about the mifepristone, including how it works and how common it is.
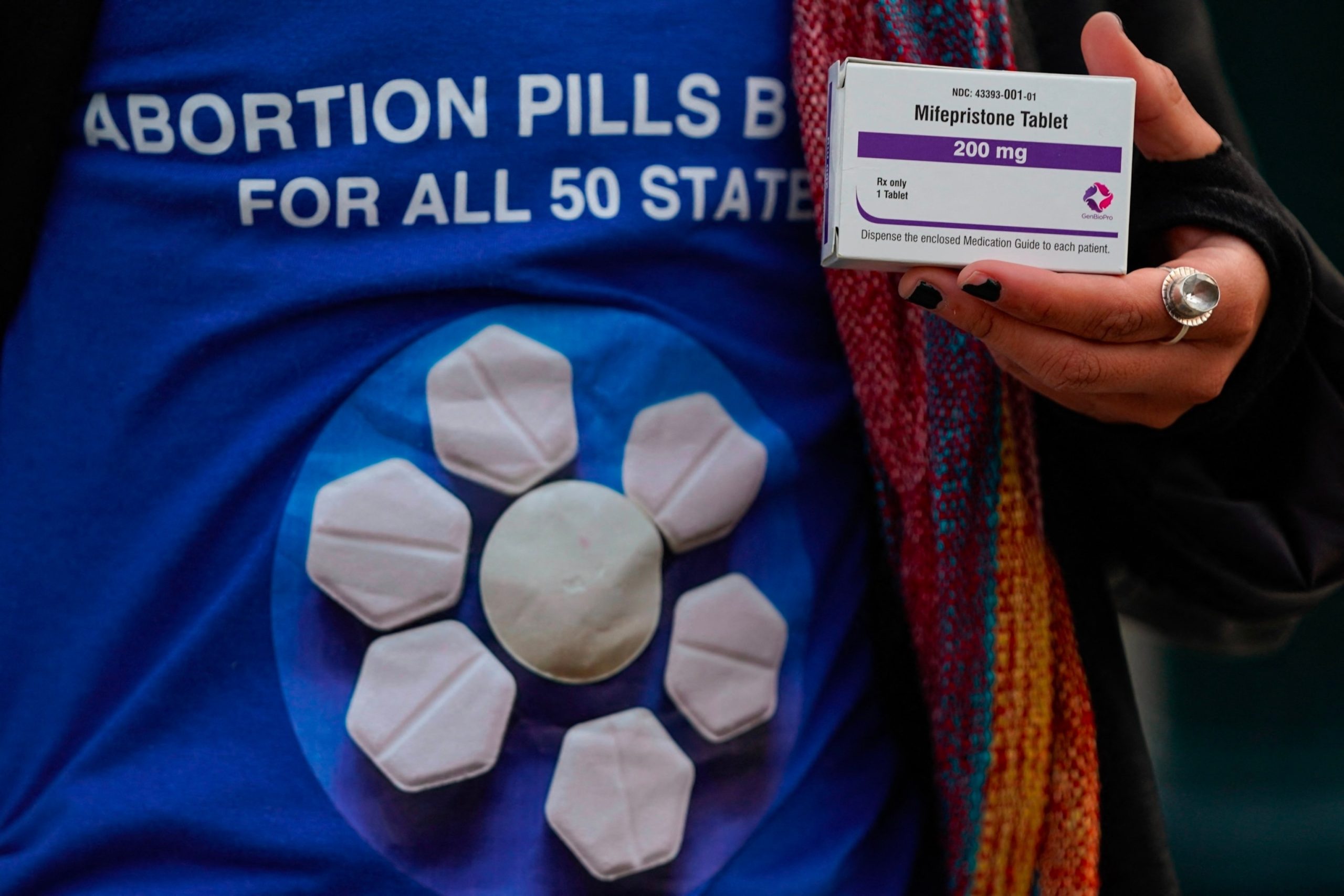
Mifepristone is an oral drug typically used in combination with another drug, misoprostol, to induce an abortion or to help manage an early miscarriage.
The medication works by blocking progesterone, a hormone that the body needs to continue a pregnancy.
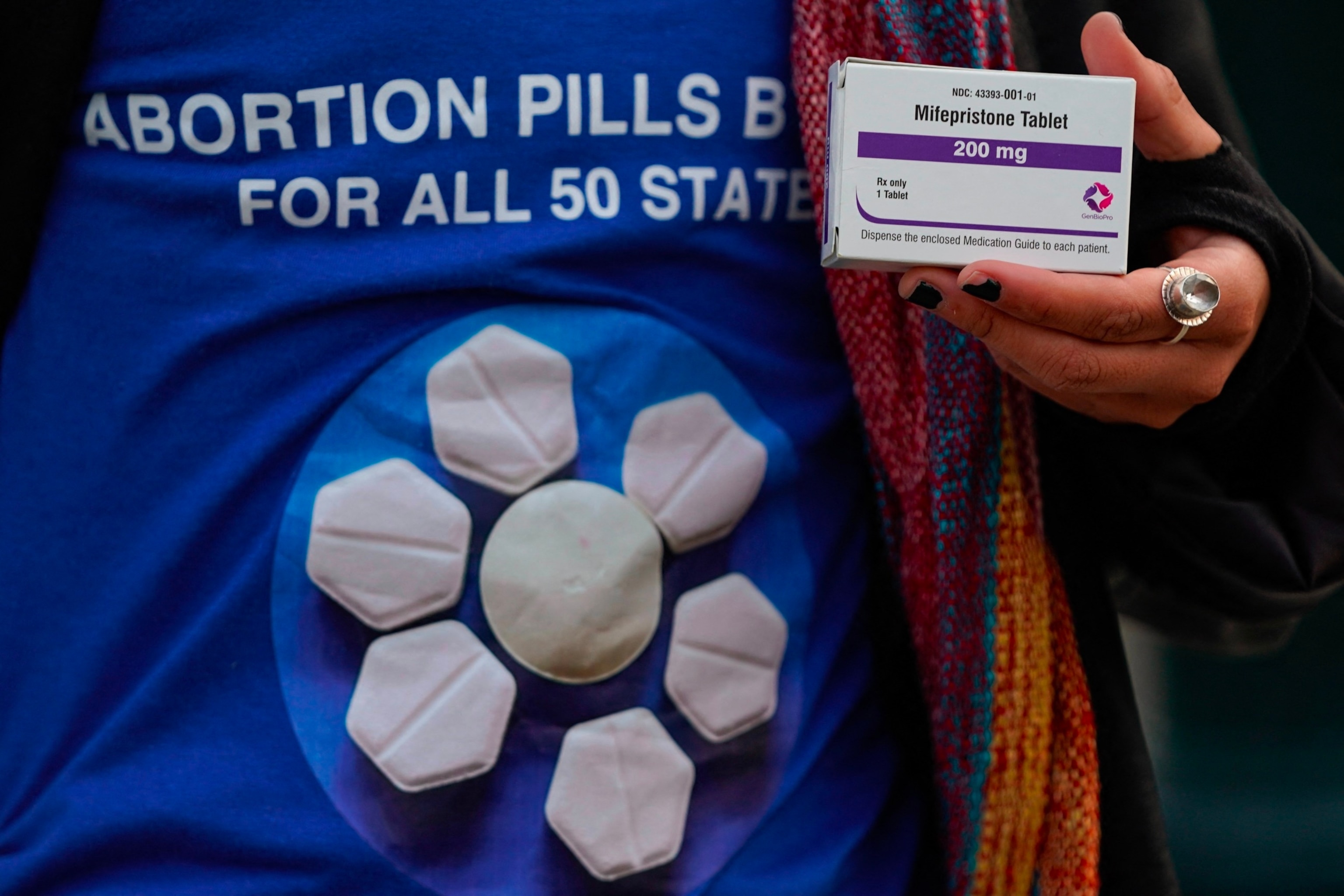
A pro-abortion rights activist holds a box of mifepristone during a rally in front of the US Supreme Court on March 26, 2024, in Washington, DC.
Drew Angerer/AFP via Getty Images
This causes the uterine lining to stop thickening and to break down, detaching the embryo. The second drug, misoprostol, taken 24 to 48 hours later, causes the uterus to contract and dilates the cervix, which will expel the embryo.
The FDA authorized mifepristone — sometimes called by the brand name Mifeprex — for medication abortion in September 2000 for up to seven weeks’ gestation, which was then extended to 10 weeks’ gestation in 2016.
However, the World Health Organization says the two drug-regimen can be taken up until the 12-week mark of pregnancy.
In 2019, the FDA approved a generic version of the drug.
Medication abortion now accounts for more than half of all abortions in the U.S., according to the Guttmacher Institute, a research group focusing on sexual and reproductive health.
As of 2023, medication abortions account for 63% of abortions performed in the U.S., up from 24% in 2011, the data from Guttmacher shows.
Mifepristone is safe and effective when used as indicated and directed by the FDA, the agency said. When used in combination, mifepristone and misoprostol are nearly 97% effective at terminating a pregnancy, according to a 2015 systematic review from the University of California, Davis.
In December 2021, the FDA announced it permanently lifted its restriction that abortion pills had to be dispensed in-person. In January 2023, it went further by allowing retail pharmacies to provide the drug too, either by mail or in person, so long as they meet certain requirements.
Mifepristone is not recommended if a woman is more than 70 days out from their last menstrual period or if they have an ectopic pregnancy, have problems with glands near the kidney, have bleeding problems, are taking blood thinning medication, have an IUD in place or have had an allergic reaction to mifepristone.
On December 10, 2021, the United States Supreme Court issued a ruling that upheld a lower court decision allowing for the distribution of mifepristone, also known as the abortion pill, through mail or delivery services during the COVID-19 pandemic. This decision has significant implications for women’s access to safe and effective abortion care in the United States.
Mifepristone is a medication used in combination with another drug, misoprostol, to induce a medical abortion up to 10 weeks into a pregnancy. It has been approved by the Food and Drug Administration (FDA) since 2000 and has been proven to be a safe and effective method of terminating a pregnancy.
Prior to the Supreme Court ruling, the FDA had imposed restrictions on the distribution of mifepristone, requiring it to be dispensed in person at a medical facility, such as a doctor’s office or clinic. This requirement posed significant barriers to access for many women, particularly those living in rural areas or facing transportation challenges.
The COVID-19 pandemic further exacerbated these barriers, as many medical facilities were forced to limit in-person services to prevent the spread of the virus. In response to these challenges, several states and medical organizations filed lawsuits challenging the FDA’s restrictions on mifepristone distribution.
The Supreme Court’s ruling effectively lifts these restrictions, allowing for mifepristone to be distributed through mail or delivery services for the duration of the pandemic. This decision is a major victory for reproductive rights advocates and will help ensure that women have access to safe and effective abortion care during this challenging time.
It is important for women to be aware of their rights and options when it comes to accessing mifepristone. If you are considering a medical abortion, it is recommended that you consult with a healthcare provider to determine if it is a safe and appropriate option for you.
It is also important to be aware of the potential side effects and risks associated with mifepristone. Common side effects may include cramping, bleeding, nausea, and diarrhea. In rare cases, serious complications such as infection or incomplete abortion may occur.
If you experience severe or persistent symptoms after taking mifepristone, it is important to seek medical attention immediately. Your healthcare provider can provide guidance on how to manage any side effects and ensure that you receive appropriate follow-up care.
Overall, the Supreme Court’s ruling on mifepristone is a positive step towards expanding access to safe and effective abortion care in the United States. By being informed about your options and rights, you can make empowered decisions about your reproductive health.