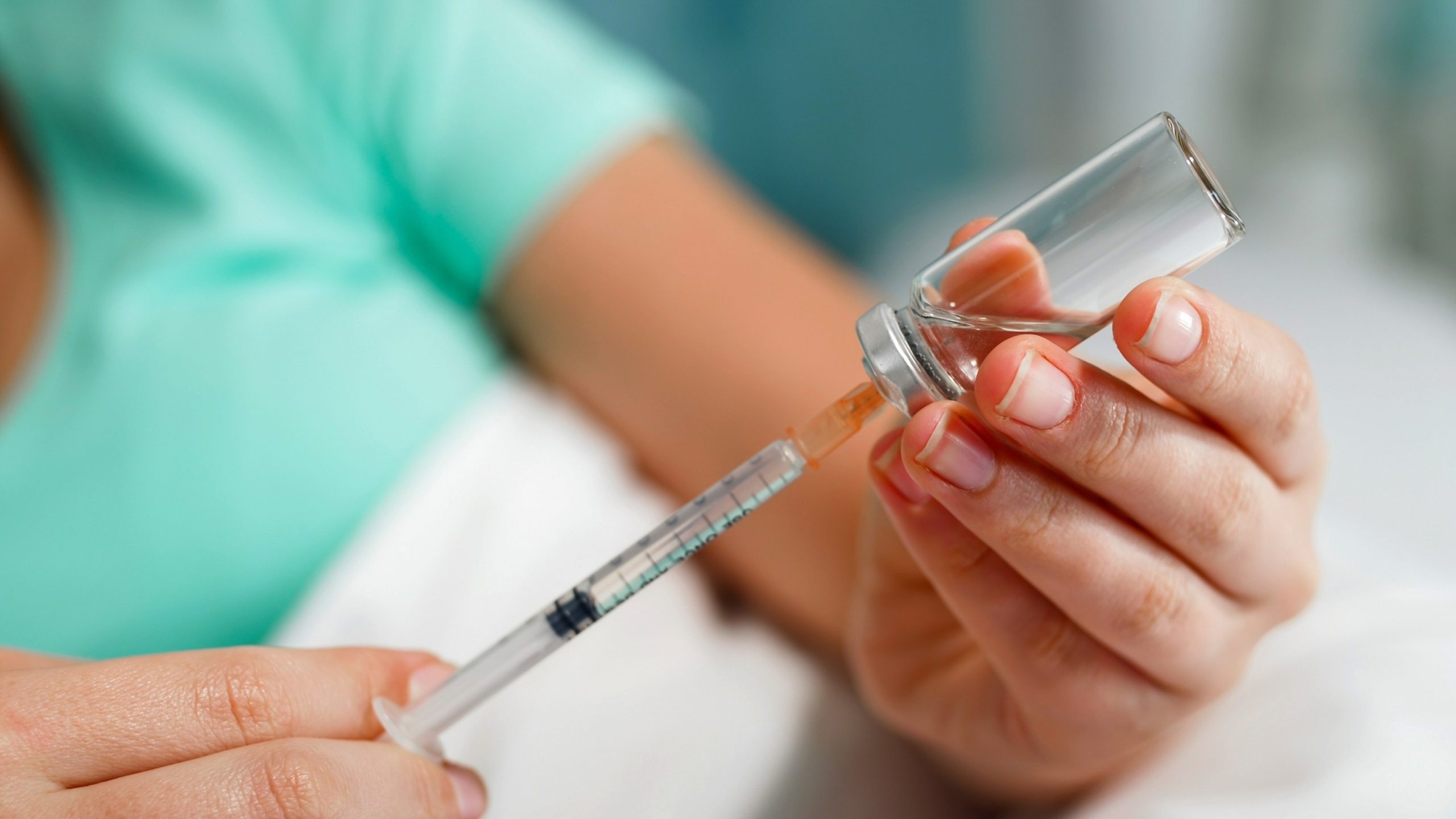
The U.S. Food and Drug Administration has issued a new warning about a popular medication used for weight loss.
The FDA says it has received reports of people overdosing on compounded semaglutide, giving themselves as much as 20 times more than the intended dose of the medication.
The incidents, some of which required hospitalization, involve semaglutide that is drawn from a vial and taken by injection, according to the FDA.
The agency said the dosing errors are a result of both patients measuring and self-administering incorrect doses of the medication, as well as health care providers “miscalculating” doses of the medication.
“Many of the patients who received vials of compounded semaglutide lacked experience with self-injections, according to the adverse event reports,” the FDA said in a July 26 statement. “Unfamiliarity with withdrawing medication from a vial into a syringe and coupled with confusion between different units of measurement (e.g., milliliters, milligrams and “units”) may have contributed to dosing errors.”
The agency said patients should consult with a medical professional on how to measure and administer the correct dose, and that health care providers should “provide patients with the appropriate syringe size for the intended dose and counsel patients on how to measure the intended dose using the syringe.”
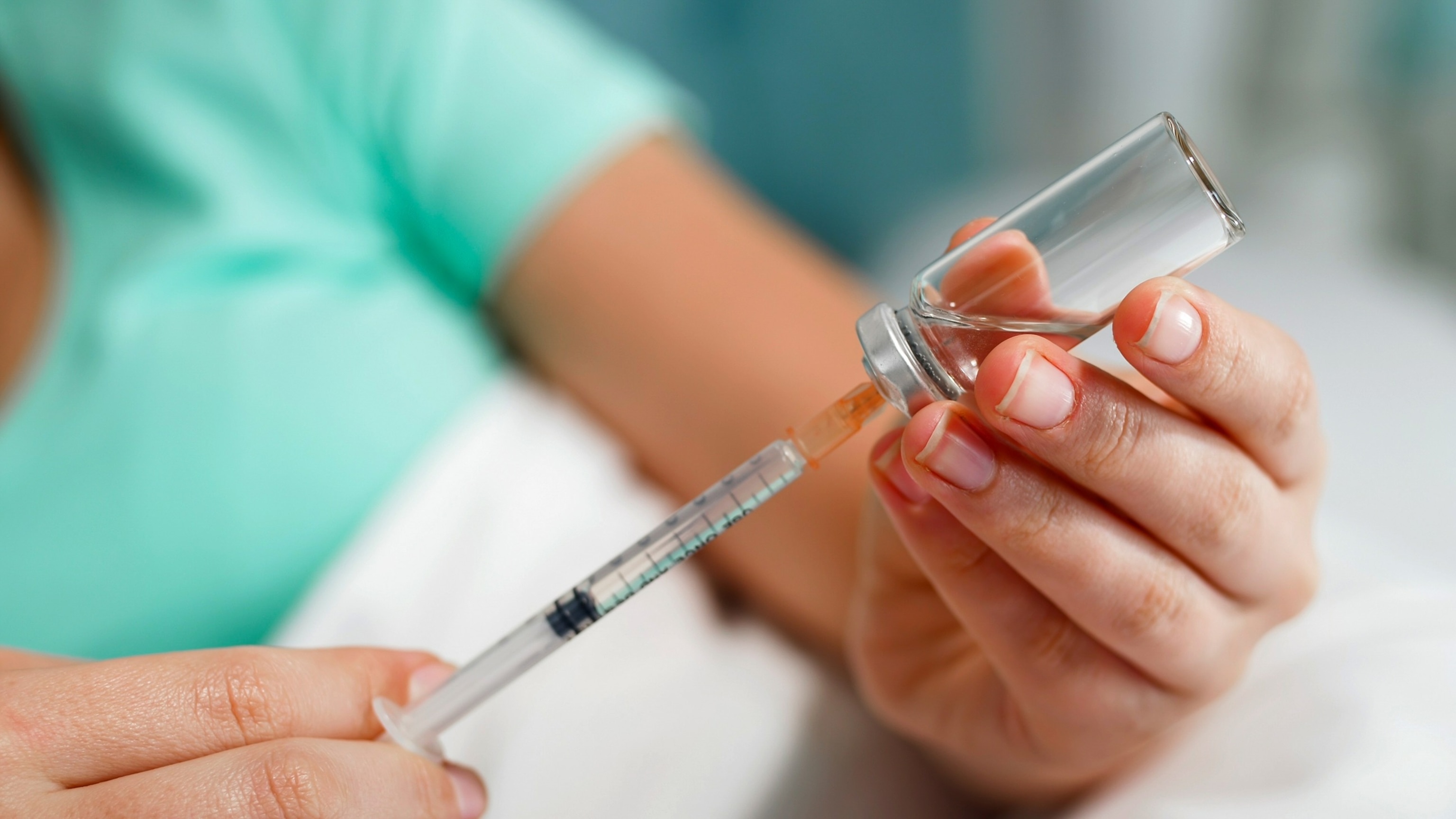
STOCK PHOTO/Getty Images
Semaglutide is the active ingredient in three FDA-approved medications, Wegovy, Ozempic and Rybelsus.
While the FDA-approved medications are administered via either an oral tablet or pre-filled pens, compounded versions of semaglutide have patients draw the dose themselves from vials, which can lead to dosing errors.
Editor’s Picks
Overdose symptoms of compounded semaglutide include nausea, vomiting, abdominal pain, fainting, headache, migraine, dehydration, acute pancreatitis and gallstones, according to the FDA.
People should contact their health care provider if they feel any symptoms.
The FDA is also encouraging people to report “adverse events” and “medication errors” to the agency via its online portal or by downloading and faxing an adverse event report.
The FDA has previously warned against the use of compounded semaglutide, citing safety concerns.
Compound pharmacies create their own semaglutide or tirzepatide compounds using raw ingredients. They are not the same as generic drugs, which are FDA-approved and monitored for safety and effectiveness.
There are currently no generic versions of semaglutide medications, including Ozempic and Wegovy.
“Patients should not use a compounded drug if an approved drug is available to treat a patient. Patients and health care professionals should understand that the agency does not review compounded versions of these drugs for safety, effectiveness, or quality,” the FDA said in a safety warning earlier this year.
The warning came as many people reported turning to compounding pharmacies to get cheaper doses of semaglutide.
Without insurance coverage, the cost of medications like Ozempic, Rybelsus and Wegovy can run more than $1,000 a month.
Both Ozempic and Rybelsus are approved by the FDA to treat Type 2 diabetes, but some doctors prescribe the medication “off-label” for weight loss, as is permissible by the FDA.
Wegovy is FDA-approved for weight loss for people with obesity or who are overweight with a comorbidity like high blood pressure.
The Food and Drug Administration (FDA) has issued a warning about the potential for overdosing on the medication semaglutide. Semaglutide is a prescription medication used to treat type 2 diabetes by helping to control blood sugar levels. It is also used in higher doses to help with weight loss.
The FDA’s warning comes after reports of patients accidentally overdosing on semaglutide, leading to serious health complications. Overdosing on semaglutide can cause symptoms such as nausea, vomiting, diarrhea, abdominal pain, and low blood sugar levels. In severe cases, it can lead to dehydration, kidney damage, and even death.
It is important for patients taking semaglutide to follow their healthcare provider’s instructions carefully and to never take more than the prescribed dose. Patients should also be aware of the signs of overdose and seek medical attention immediately if they experience any symptoms.
In addition to the risk of accidental overdose, patients should also be cautious about taking other medications that may interact with semaglutide. Certain medications, such as insulin or sulfonylureas, can increase the risk of low blood sugar levels when taken with semaglutide.
Patients should always inform their healthcare provider about all medications they are taking, including over-the-counter medications and supplements, to avoid any potential interactions.
The FDA recommends that healthcare providers closely monitor patients taking semaglutide for any signs of overdose or adverse reactions. Patients should also be educated on the proper use of the medication and the importance of following dosing instructions carefully.
Overall, while semaglutide can be an effective treatment for type 2 diabetes and weight loss, it is important for patients to be aware of the potential risks of overdosing and to take precautions to ensure their safety. By following their healthcare provider’s instructions and being vigilant about their medication use, patients can minimize the risk of complications and safely benefit from the effects of semaglutide.