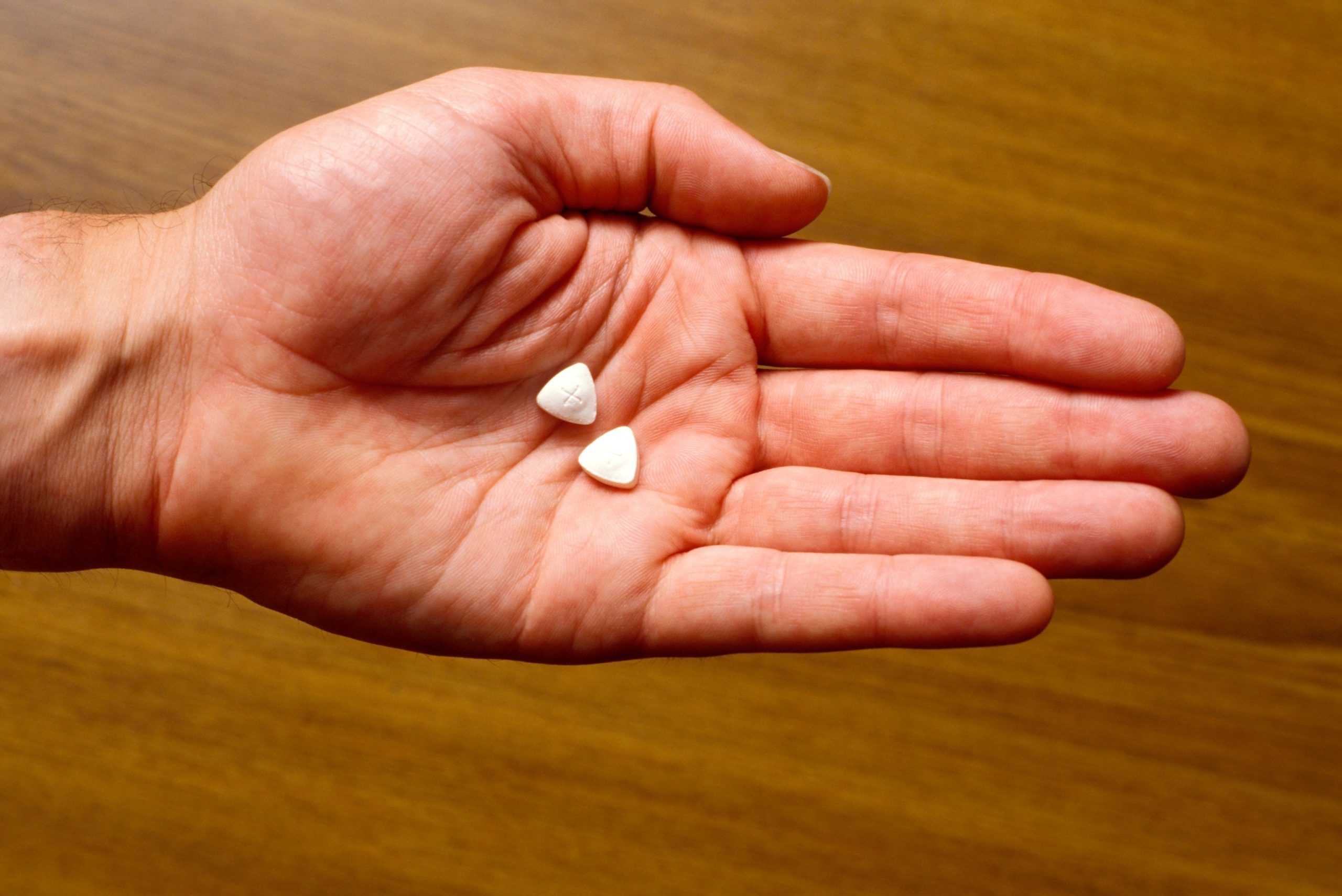
The U.S. Food and Drug Administration (FDA) has decided not to approve a psychedelic treatment — along with therapy — to treat post-traumatic stress disorder (PTSD), according to Lykos Therapeutics, the company that applied for approval.
The treatment is a pharmaceutical version of midomafetamine, better known as MDMA and sometimes referred to as ecstasy.
The FDA requested an additional Phase 3 trial. The rejection comes on the heels of controversy about Lykos’ existing clinical trial, which was plagued with accusations of misconduct. The issues raised by the FDA echo similar concerns raised in a May meeting of FDA advisors, who voted against approval.
The company said it conducted two late-stage randomized placebo-controlled trials to evaluate the safety and efficacy of MDMA when used in combination with psychological intervention, such as talk therapy, and found benefits.
A multitude of studies have suggested that psychoactive drugs, including cannabis, ketamine magic mushrooms and MDMA, may help treat PTSD and other mental health disorders.
Some psychiatrists began using MDMA for treatment in the late 1970s and early 1980s believing it helped promote trust and improved communication between doctors and patients.
MDMA was classified as a Schedule I drug, meaning it has “no currently accepted medical use and a high potential for abuse,” by the Drug Enforcement Administration in 1985.
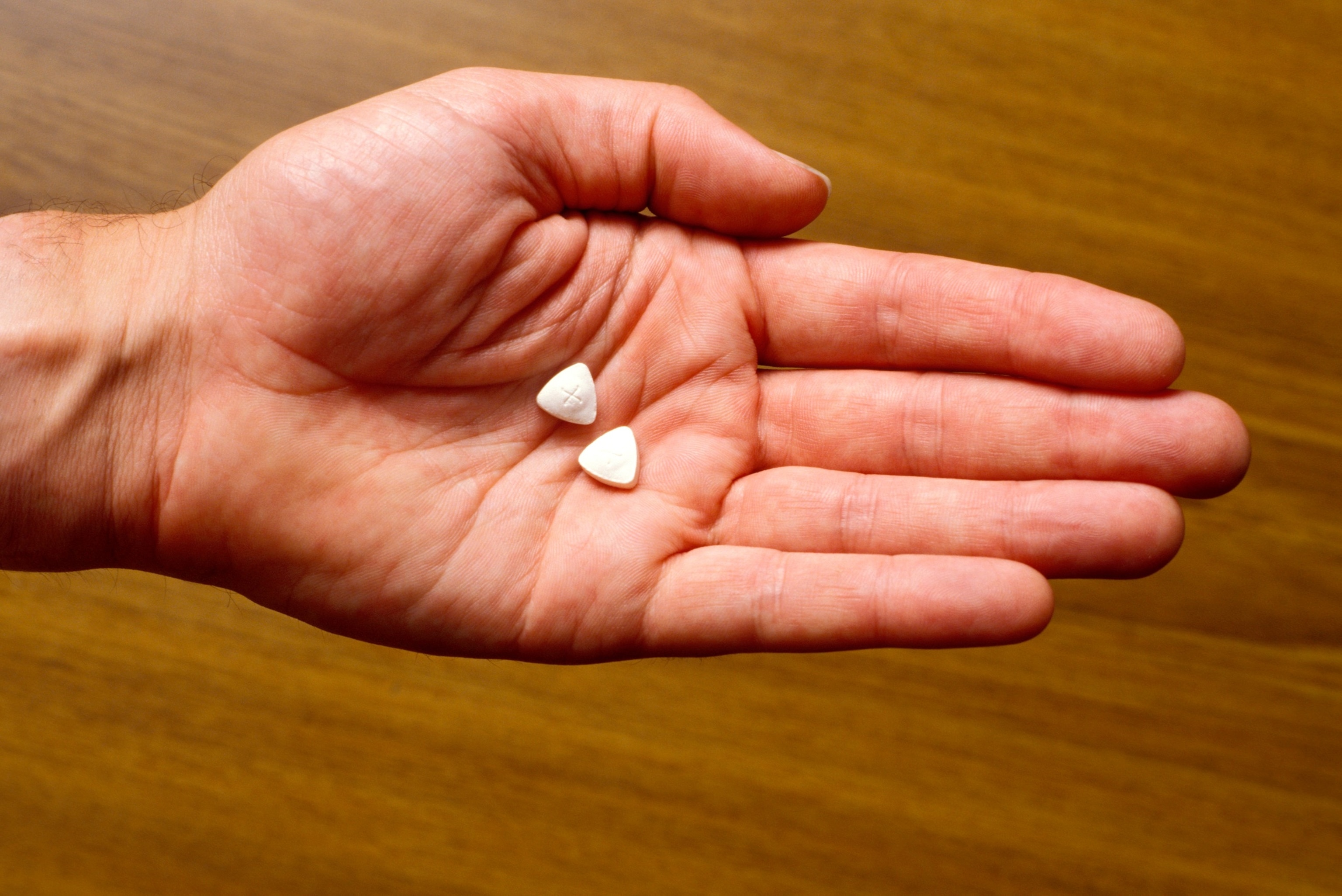
Ecstasy pills in hand.
Pymca/avalon/Avalon via Getty Images
The FDA had until Aug. 11 to make a decision on approval.
The FDA’s decision comes after a panel of independent advisers for the federal agency voted against recommending approval of the treatment during a meeting in early June.
The Psychopharmacologic Drugs Advisory Committee appeared to have serious doubts about the safety and efficacy of the treatment.
In briefing documents released earlier this year, the FDA said that based on the clinical trial data submitted by Lykos Therapeutics, “participants appear to experience rapid, clinically meaningful, durable improvement in their PTSD symptoms.”
Despite the concerns of health officials, lawmakers had urged the FDA to approve the application, with more than 60 members of Congress signing a letter to President Joe Biden expressing their desire for the FDA to approve the treatment.
The FDA rejecting the application “doesn’t necessarily mean the end of psychedelic drug development, since several of the issues of concerns about the application are related to the fact that the effort to get MDMA approved has until recently been a grassroots effort…rather than a pharmaceutical company with seasoned industry veterans,” Dr Brian Barnett, clinical director of the Cleveland Clinic psychiatric treatment resistance program, told ABC News in a statement. “The path toward FDA approval has been really unique in this regard.”
ABC News’ Dr. Nicholas Nissen contributed to this report.
The Food and Drug Administration (FDA) has not approved the use of MDMA, also known as ecstasy, in combination with therapy for the treatment of post-traumatic stress disorder (PTSD). Despite promising results from clinical trials, the FDA has not yet given the green light for this unconventional treatment approach.
MDMA is a psychoactive drug that is known for its ability to produce feelings of euphoria, empathy, and emotional openness. In recent years, researchers have been exploring the potential therapeutic benefits of MDMA in combination with therapy for individuals suffering from PTSD. Studies have shown that MDMA-assisted therapy can help patients process traumatic memories and emotions in a more effective and efficient way.
One of the most notable studies on MDMA-assisted therapy for PTSD was conducted by the Multidisciplinary Association for Psychedelic Studies (MAPS). The study found that participants who received MDMA-assisted therapy experienced significant reductions in PTSD symptoms compared to those who received therapy without MDMA. These results have sparked interest in using MDMA as a tool to enhance the effectiveness of traditional therapy for PTSD.
Despite these promising findings, the FDA has not yet approved MDMA for the treatment of PTSD. The agency has raised concerns about the potential risks and side effects associated with the use of MDMA, including increased heart rate, elevated blood pressure, and potential neurotoxicity. Additionally, there are concerns about the potential for abuse and addiction with MDMA, which is classified as a Schedule I controlled substance by the Drug Enforcement Administration.
While researchers continue to explore the potential benefits of MDMA-assisted therapy for PTSD, it is important to proceed with caution. More research is needed to fully understand the long-term effects and risks associated with this treatment approach. In the meantime, individuals suffering from PTSD should consult with their healthcare providers to explore other evidence-based treatment options that have been approved by the FDA.
In conclusion, while MDMA-assisted therapy shows promise as a potential treatment for PTSD, the FDA has not yet approved this approach due to concerns about safety and potential risks. It is important for researchers to continue studying the effects of MDMA on PTSD and for patients to work with their healthcare providers to explore other treatment options that have been proven to be safe and effective.