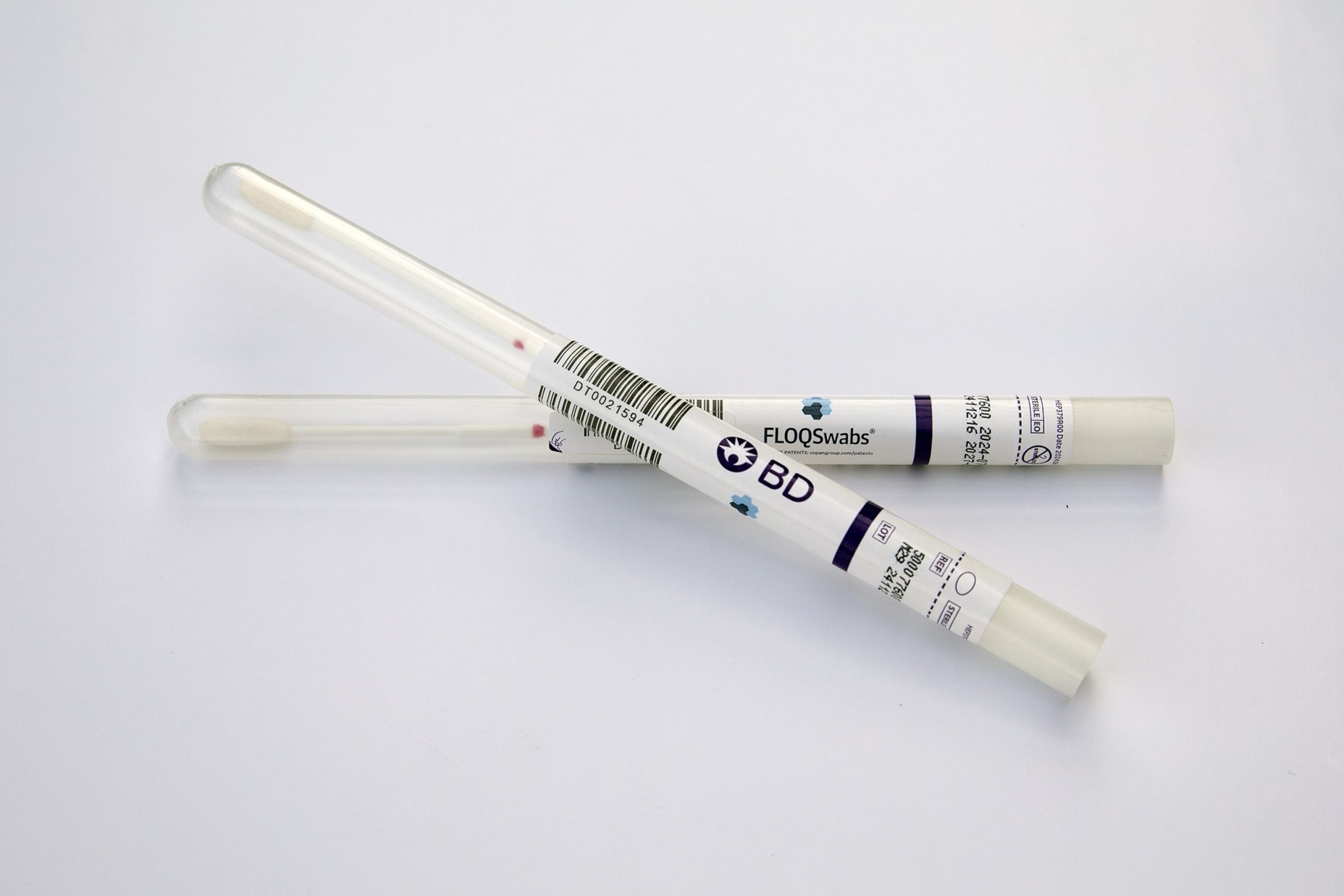
One of the first tests that allows patients to self-collect samples to screen for human papillomavirus (HPV) will soon be available in doctors’ offices.
In May, the U.S. Food and Drug Administration (FDA) approved a self-swab test from global medical technology company Becton, Dickinson and Company (BD) for “clinical” use, meaning in a private room inside a doctor’s office, in a mobile clinic, or in another health care setting.
BD told ABC News that it started shipping its self-swab kits, called the BD Onclarity HPV Assay, to doctors’ offices on Thursday and that kits will begin arriving at health care facilities later this month.
HPV is the most common sexually transmitted infection in the U.S. and can lead to several potentially deadly cancers, including cervical cancer, according to the Centers for Disease Control and Prevention. HPV causes some 36,000 cases of cancer in men and women in the U.S. every year, the CDC says.
Typically, screening for HPV in patients involves a Pap smear, also known as a Pap test. A small brush is used to gently remove cells from the surface of the cervix and the area around the cervix. The swab is then checked under a microscope for signs of cervical cancer or cell changes that could lead to cervical cancer.
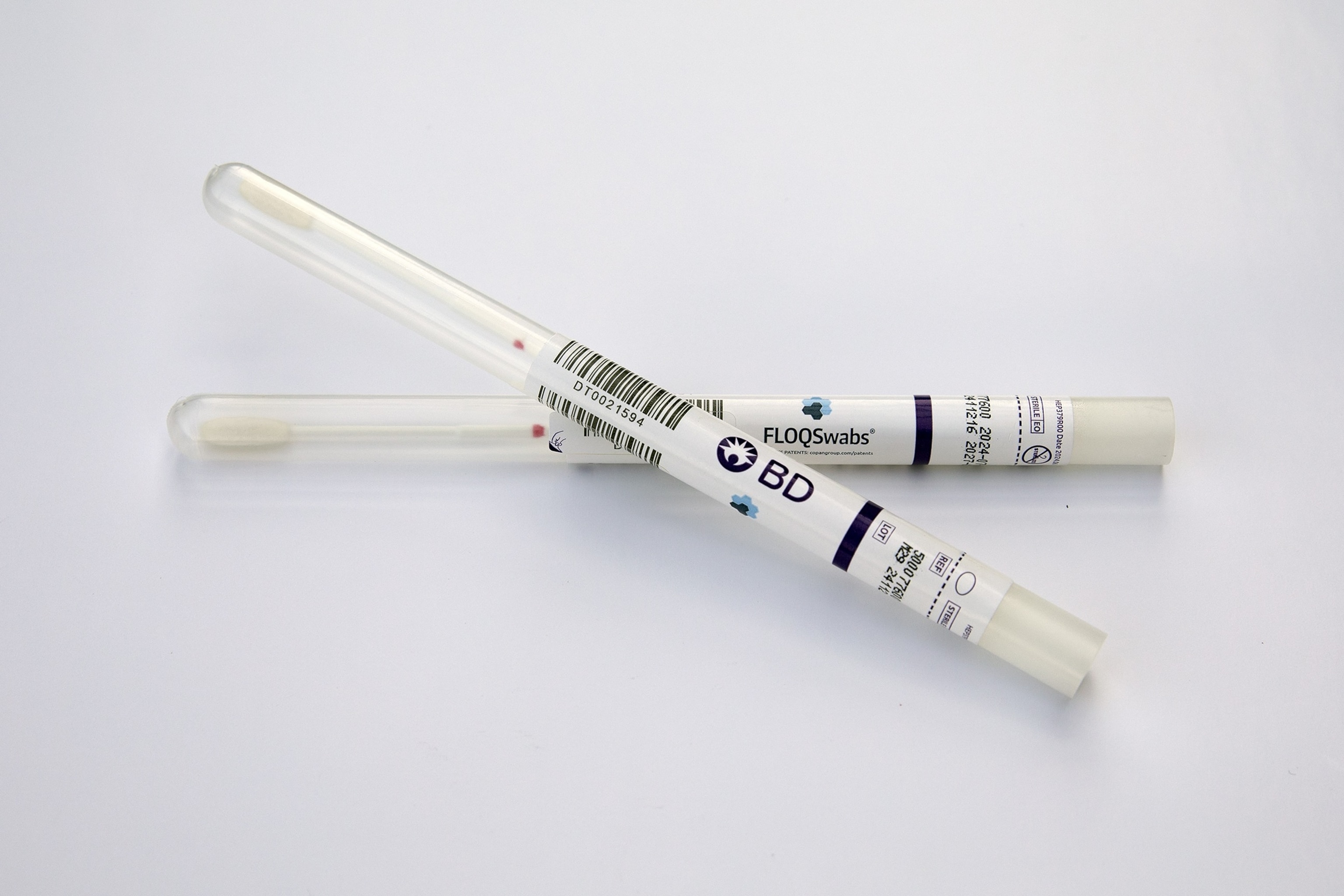
The BD self-swab test will soon be made available in doctors’ offices.
BD
In a press release, BD noted that many people are not comfortable with the pelvic exam required for a traditional Pap smear for a variety of reasons, including past negative experiences, or social or religious preferences. The company also noted that traditional exams may include people having to place their legs in medical stirrups, or doctors choosing to use speculums.
The new tests will allow patients to insert the swab and collect the sample themselves, even at a primary care doctor’s office. The swabs are then sent to a lab, which will relay results to the ordering doctor, who will then update the patient.
By having a less-intrusive option, BD says more people, especially those living in medically underserved areas, can get screened for HPV and potentially prevent cervical cancer.
“Over half of cervical cancers occur in women who have not been screened in the last five years or who have never been screened,” Dr. Jeff Andrews, a board-certified gynecologist and vice president of global medical affairs for diagnostic solutions at BD, told ABC News in a statement. “Self-collection changes the conversation about screening and is an important step forward toward the elimination of cervical cancer in our lifetimes. It’s simple, private and easy to use, all of which can help those who, for a variety of reasons from socioeconomic to personal, haven’t been screened get access to potentially life-saving testing.”
Also in May, the FDA approved a similar HPV self-collection test from pharmaceutical company Roche Holding AG. Roche told ABC News it does not yet have an update regarding when their test will be available.
BD has said that it eventually wants to make its HPV self-screening test available for at-home use, adding that it could happen by the end of the year, pending FDA approval specifically for at-home collection.
Starting this month, doctors’ offices across the country will begin offering self-swab HPV tests to patients. This new testing option is a game-changer for individuals who may be hesitant to undergo traditional HPV testing, which often involves a pelvic exam.
HPV, or human papillomavirus, is a common sexually transmitted infection that can lead to cervical cancer if left untreated. It is estimated that nearly 80 million Americans are currently infected with HPV, and many may not even know it due to the lack of symptoms.
The self-swab HPV test allows individuals to collect their own sample in the privacy of their own home or a doctor’s office restroom. This simple and convenient test involves using a small swab to collect cells from the vagina or cervix, which are then sent to a lab for analysis.
By offering self-swab HPV tests, doctors’ offices hope to increase screening rates and ultimately prevent more cases of cervical cancer. Studies have shown that self-collected samples are just as accurate as those collected by a healthcare provider, making this new testing option a reliable and effective tool in the fight against HPV-related cancers.
In addition to convenience, self-swab HPV tests also offer a sense of empowerment to individuals who may feel uncomfortable or anxious about traditional testing methods. By allowing patients to take control of their own health and participate in the screening process, doctors hope to reduce barriers to testing and encourage more people to get screened for HPV.
It is important to note that while self-swab HPV tests are a valuable screening tool, they are not a replacement for regular Pap smears or HPV tests conducted by a healthcare provider. Individuals should still follow their doctor’s recommendations for cervical cancer screening and discuss any concerns or symptoms with their healthcare provider.
Overall, the introduction of self-swab HPV tests in doctors’ offices marks a significant step forward in the fight against cervical cancer. By making testing more accessible and empowering individuals to take charge of their own health, we can work towards reducing the burden of HPV-related cancers and improving outcomes for all patients.