The US Food and Drug Administration (FDA) has recently approved the first-ever vaccine for respiratory syncytial virus (RSV) for older adults. This is a significant milestone in the field of medicine, as RSV is a common respiratory illness that can be particularly dangerous for older adults.
RSV is a highly contagious virus that affects the respiratory system, causing symptoms such as coughing, wheezing, and difficulty breathing. While most people recover from RSV without any serious complications, it can be life-threatening for older adults, young children, and people with weakened immune systems.
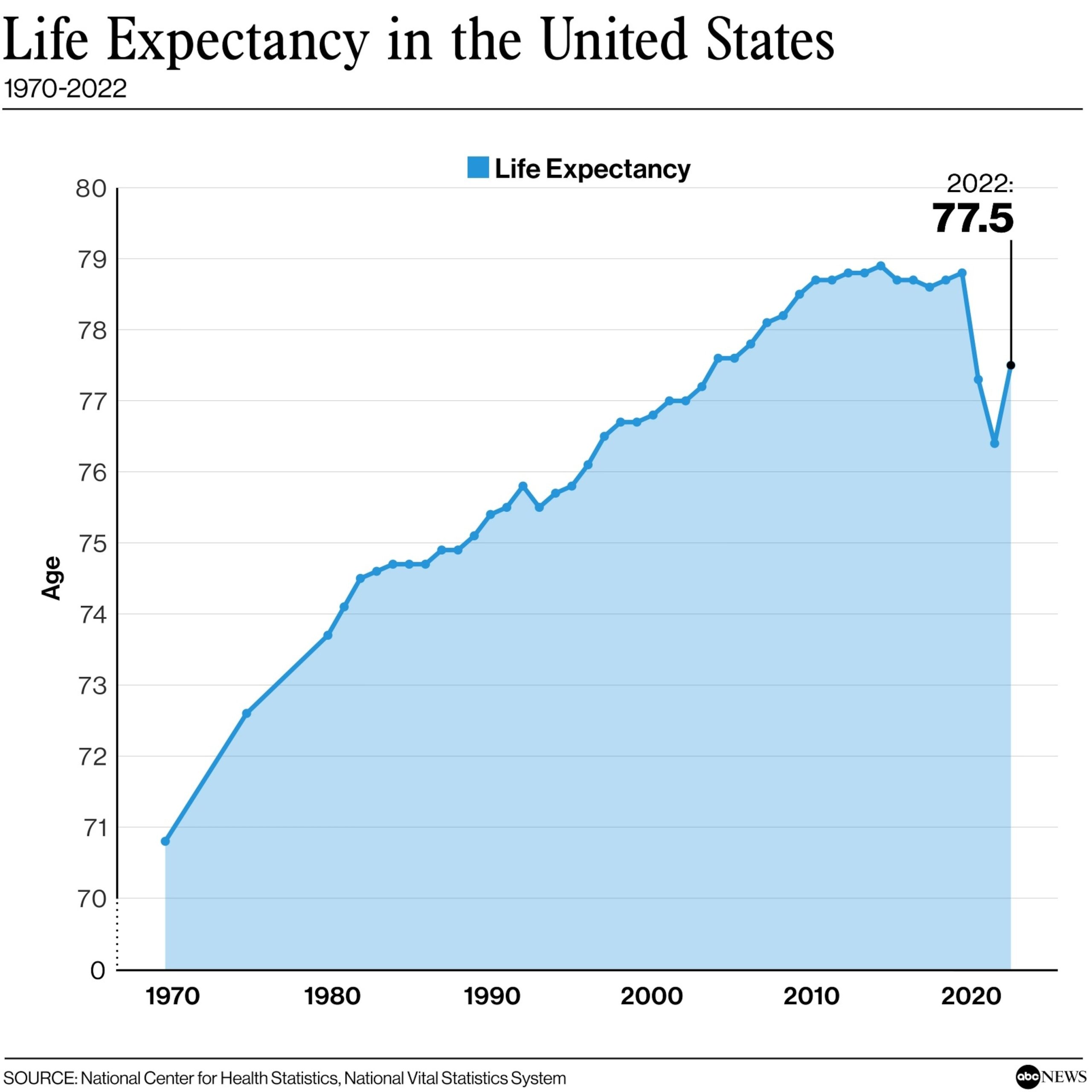
According to the Centers for Disease Control and Prevention (CDC), RSV is responsible for up to 177,000 hospitalizations and 14,000 deaths among older adults in the US each year. This makes it a serious public health concern, particularly as the population ages.
The new RSV vaccine, called Synagis (palivizumab), is designed to prevent severe RSV infections in older adults. It works by targeting a specific protein on the surface of the virus, preventing it from entering and infecting cells in the respiratory system.
Synagis has been in use for several years as a preventive treatment for RSV in infants and young children. However, this is the first time it has been approved for use in older adults.
The FDA’s decision to approve Synagis for older adults is based on the results of a clinical trial involving over 1,600 participants aged 60 and older. The trial found that the vaccine reduced the incidence of severe RSV infections by 63% compared to a placebo.
This is a significant breakthrough in the field of medicine, as there are currently no other vaccines available to prevent RSV infections in older adults. The approval of Synagis could potentially save thousands of lives each year and reduce the burden on healthcare systems.
However, it’s important to note that Synagis is not a cure for RSV and will not prevent all cases of the virus. It is also a relatively expensive treatment, with a cost of around $3,000 per dose.
Despite these limitations, the approval of Synagis is a historic moment in the fight against RSV. It represents a significant step forward in our ability to prevent and treat this common respiratory illness, particularly in older adults who are most at risk. As further research is conducted, it’s possible that we may see even more effective vaccines and treatments for RSV in the future.