The Food and Drug Administration (FDA) has recently granted approval for the first maternal respiratory syncytial virus (RSV) vaccine, marking a significant milestone in protecting infants from this common respiratory infection. RSV is a leading cause of severe respiratory illness in young children, particularly those under the age of one. This groundbreaking vaccine aims to provide passive immunity to newborns by immunizing pregnant women.
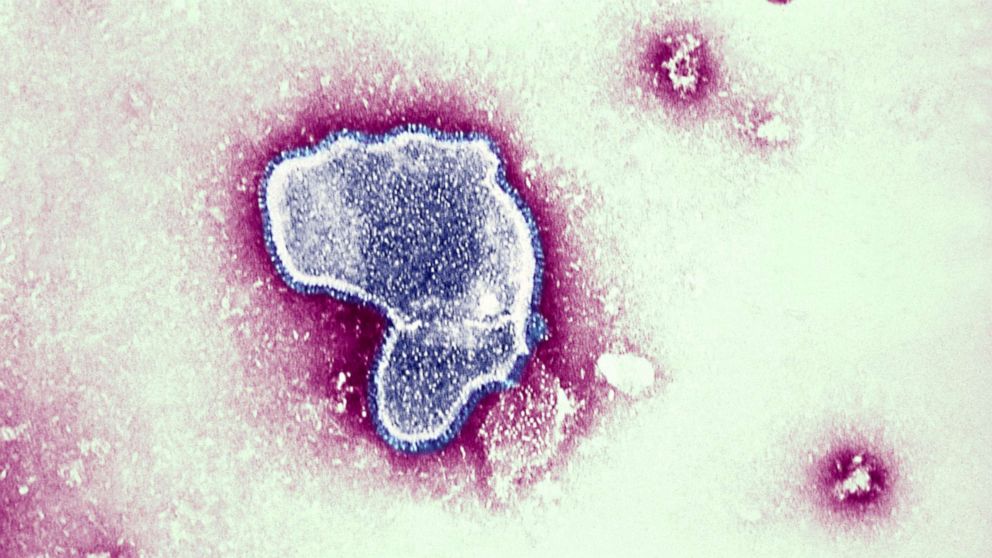
RSV is a highly contagious virus that affects the respiratory system, causing symptoms such as coughing, sneezing, runny nose, and fever. While most healthy individuals recover from RSV with mild symptoms, it can be life-threatening for infants, especially those born prematurely or with underlying health conditions. Each year, RSV leads to numerous hospitalizations and even deaths among infants worldwide.
The newly approved vaccine, known as RSVpreF, is administered to pregnant women during their third trimester. By vaccinating expectant mothers, the aim is to stimulate the production of protective antibodies that can be transferred to their unborn babies through the placenta. These antibodies will then provide passive immunity to the infants during the first few months of life when they are most vulnerable to severe RSV infections.
Clinical trials have shown promising results for RSVpreF. In a study involving over 4,600 pregnant women, the vaccine was found to be safe and effective in preventing severe RSV-associated lower respiratory tract infections in infants. The vaccinated infants had a significantly lower rate of hospitalization due to RSV compared to those born to mothers who did not receive the vaccine.
The approval of this maternal RSV vaccine is a significant step forward in protecting infants from this potentially dangerous infection. Prior to this, there were no approved vaccines specifically targeting RSV in infants. The availability of this vaccine offers hope for reducing the burden of RSV-related hospitalizations and improving outcomes for vulnerable infants.
It is important to note that while the vaccine provides passive immunity to newborns, it does not protect pregnant women from RSV infection. Therefore, it is crucial for pregnant women and those around them to take necessary precautions to prevent RSV transmission, such as practicing good hand hygiene, avoiding close contact with sick individuals, and staying away from crowded places during peak RSV season.
The approval of the first maternal RSV vaccine is a significant achievement in the field of pediatric immunization. It represents a major step forward in safeguarding infants from severe respiratory infections caused by RSV. With further research and development, it is hoped that additional vaccines and preventive measures will be available to protect children from other common respiratory viruses as well. In the meantime, healthcare providers and public health organizations can now offer this important vaccine to pregnant women, providing them with an effective tool to protect their newborns from the potentially devastating effects of RSV.