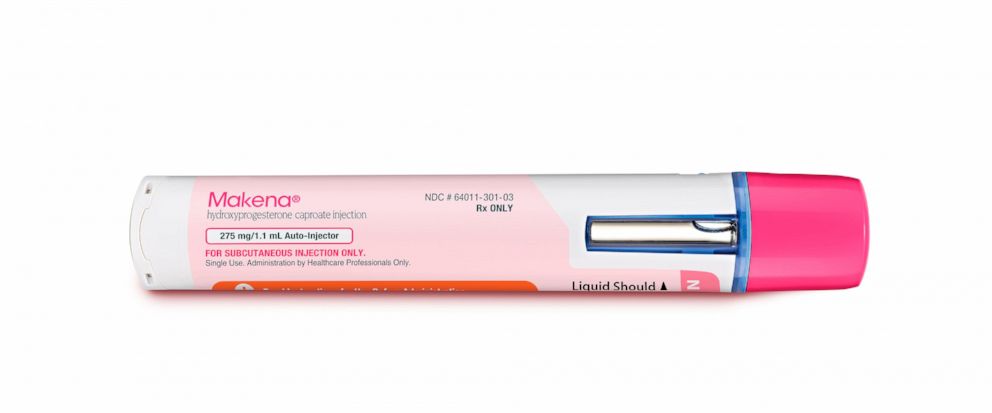
Makena, an unproven premature birth drug, is being removed from the market by the FDA. This decision comes after years of controversy over the drug’s effectiveness and safety.
Makena, also known as hydroxyprogesterone caproate, was approved by the FDA in 2011 to reduce the risk of preterm birth in women who had previously given birth prematurely. The drug was marketed by AMAG Pharmaceuticals and sold for $20,000 per treatment.
However, Makena’s effectiveness has been called into question by several studies. In 2019, a clinical trial funded by the National Institutes of Health found that Makena did not significantly reduce the rate of preterm birth compared to a placebo. This study contradicted earlier research that had suggested Makena was effective.
The controversy over Makena’s effectiveness has been compounded by concerns about its safety. In 2017, the FDA issued a warning about Makena after a study found that it may be associated with an increased risk of miscarriage and stillbirth.
Despite these concerns, AMAG Pharmaceuticals continued to market and sell Makena. In 2019, the company was acquired by Covis Pharma, which continued to sell the drug.
Now, the FDA has announced that it is removing Makena from the market. In a statement, the agency said that it had “concluded that the available evidence does not support continued marketing of Makena.”
The decision to remove Makena from the market has been welcomed by many experts in the field of obstetrics and gynecology. Dr. Laura Jelliffe-Pawlowski, director of precision health and discovery at the University of California, San Francisco, told NPR that the decision was “long overdue.”
However, some patient advocacy groups have criticized the decision. The March of Dimes, which had previously supported the use of Makena, said in a statement that it was “disappointed” by the FDA’s decision.
The controversy over Makena highlights the challenges of developing effective treatments for preterm birth. Preterm birth is a major public health issue, affecting around 10% of all births in the United States. However, there are few effective treatments available.
Moving forward, researchers will need to continue to explore new treatments for preterm birth. This will require rigorous clinical trials to ensure that new treatments are both safe and effective. Only then can we hope to reduce the number of premature births and improve outcomes for mothers and babies.